Abstract
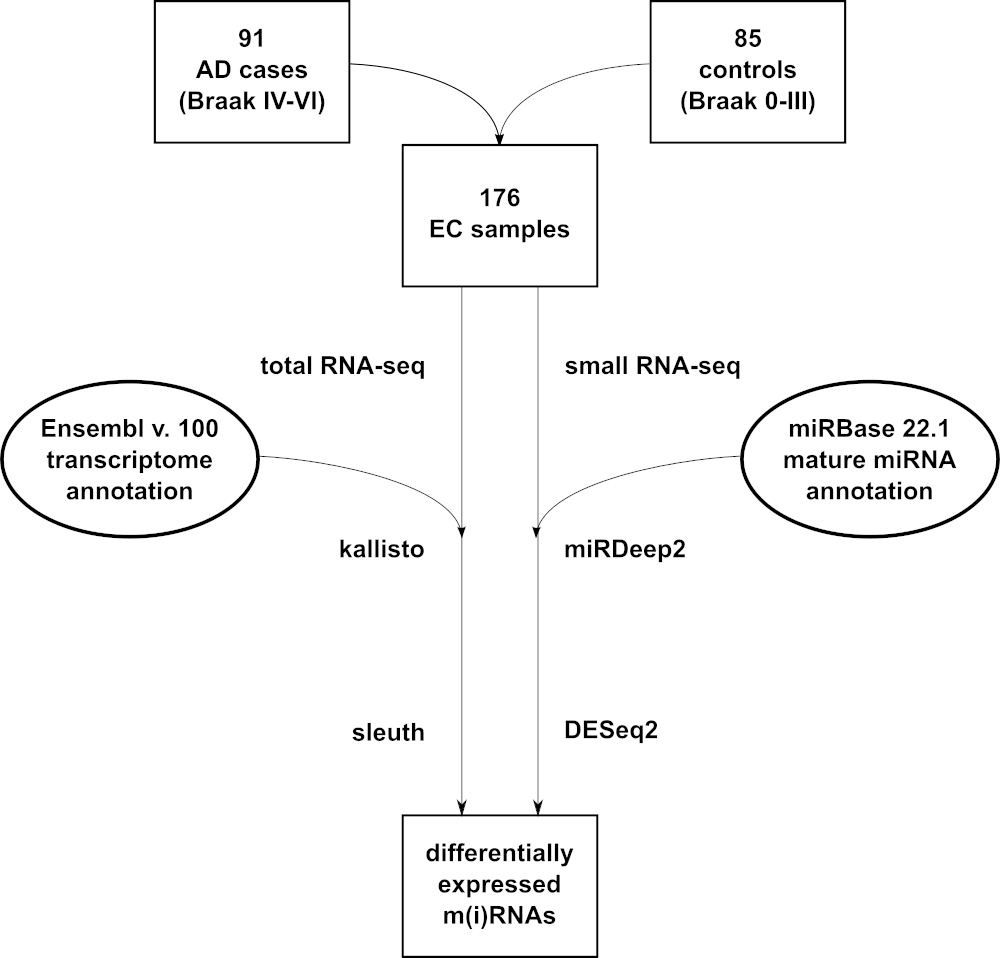
Background Dysregulation of microRNAs (miRNAs) is involved in the pathogenesis of neurodegenerative diseases, including Alzheimer’s disease (AD). Hitherto, sample sizes from differential miRNA expression studies in AD are exceedingly small aggravating any biological inference. To overcome this limitation, we investigated six candidate miRNAs in a large collection of brain samples. Methods Brain tissue was derived from superior temporal gyrus (STG) and entorhinal cortex (EC) from 99 AD patients and 91 controls. Expression of six miRNAs was examined by qPCR (STG) or small RNA sequencing (EC). Brain region-dependent differential miRNA expression was investigated in a transgenic AD mouse model using qPCR and FISH. Total RNA sequencing was used to assess differential expression of miRNA target genes. Results MiR-129-5p, miR-132-5p, and miR-138-5p were significantly downregulated in AD vs. controls both in STG and EC, while miR-125b-5p and miR-501-3p showed no evidence for differential expression in this dataset. In addition, miR-195-5p was significantly upregulated in EC but not STG in AD patients. The brain region-specific pattern of miR-195-5p expression was corroborated in vivo in transgenic AD mice. Total RNA sequencing identified several novel and functionally interesting target genes of these miRNAs involved in synaptic transmission (GABRB1), the immune-system response (HCFC2) or AD-associated differential methylation (SLC16A3). Conclusions Using two different methods (qPCR and small RNA-seq) in two separate brain regions in 190 individuals we more than doubled the available sample size for most miRNAs tested. Differential gene expression analyses confirm the likely involvement of miR-129-5p, miR-132-5p, miR-138-5p, and miR-195-5p in AD pathogenesis and highlight several novel potentially relevant target mRNAs. Funding This work was supported by the Deutsche Forschungsgemeinschaft (DFG) and the National Science Foundation China (NSFC) as a Joint Sino-German research project (“MiRNet-AD”, #391523883). Additional support was provided by the DFG Research Infrastructure NGS_CC (project 407495230) as part of the Next Generation Sequencing Competence Network (#423957469) and the Cure Alzheimer’s Fund (CAF) as part of the CIRCUITS consortium project. Competing Interest Statement The authors have declared no competing interest.